Atom economy
Atom economy (atom efficiency) describes the conversion efficiency of a chemical process in terms of all atoms involved (desired products produced). In an ideal chemical process, the amount of starting materials or reactants equals the amount of all products generated (see stoichiometry) and no atom is wasted. Recent developments like high raw material (such as petrochemicals) costs and increased sensitivity to environmental concerns have made atom economical approaches more popular. Atom economy is an important concept of green chemistry philosophy,[1][2][3] and one of the most widely used ways to measure the "greenness" of a process or synthesis.
Atom economy can be written as:
% atom economy = 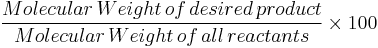
Note that atom economy can be poor even when chemical yield is near 100%, see for instance the Cannizzaro reaction or the Wittig reaction. If the desired product has an enantiomer the reaction needs to be sufficiently stereoselective even when atom economy is 100%. A Diels-Alder reaction is an example of a potentially very atom efficient reaction that also can be chemo-, regio-, diastereo- and enantioselective. Catalytic hydrogenation comes the closest to being an ideal reaction that is extensively practiced both industrially and academically.[4] The Gabriel synthesis of amines is an example of extremely low atom economy, as stochiometric quantities of phthalic acid derivatives are formed. In most cases, the atom economy of the Gabriel synthesis is <<50%.
Atom economy can also be adjusted if a pendant group is recoverable, for example Evans auxiliary groups. However, if this can be avoided it is more desirable, as recovery processes will never be 100%. Atom economy can be improved upon by careful selection of starting materials and a catalyst system.
Atom economy is just one way to evaluate a chemical process. Other criteria can include energy consumption, pollutants released and price.
Poor atom economy is common in fine chemicals or pharmaceuticals synthesis, and especially in research, where the aim to readily and reliably produce a wide range of complex compounds leads to the use of versatile and dependable, but poorly atom-economical reactions. For example, synthesis of an alcohol is readily accomplished by reduction of an ester with lithium aluminum hydride, but the reaction necessarily produces a voluminous floc of aluminum salts, which have to be separated from the product alcohol and disposed of. The cost of such hazardous material disposal can be considerable. Catalytic hydrogenolysis of an ester is the analogous reaction with a high atom economy, but it requires catalyst optimization, is a much slower reaction and is not applicable universally.
Creating reactions utilizing atom economy
It is fundamental in chemical reactions of the form A+B→ C+D that two products are necessarily generated though product C may have been the desired one. That being the case, D is considered a byproduct. As it is a significant goal of green chemistry to maximize the efficiency of the reactants and minimize the production of waste, D must either be found to have use, be eliminated or be as insignificant and innocuous as possible. With the new equation of the form A+B→C, the first step in making chemical manufacturing more efficient is the use of reactions that resemble simple addition reactions with the only other additions being catalytic materials.
References
- ^ Trost B. M. (1995). "Atom Economy. A Challenge for Organic Synthesis". Angew. Chem. Int. Ed. Engl. 34 (3): 259–281. doi:10.1002/anie.199502591.
- ^ Sheldon R. A. (2000). "Atom efficiency and catalysis in organic synthesis". Pure and Applied Chemistry 72 (7): 1233–1246. doi:10.1351/pac200072071233. http://www.iupac.org/publications/pac/2000/7207/7207pdf/7207sheldon_1233.pdf.
- ^ Atom Economy: A Green Chemistry Module.
- ^ Solaza, B.; Huguet, J.; Karpf, M. and Dreiding, A. S. (1987). "The Synthesis of (+/-)Isoptychanolide by Application of the a-Alkynone Cycilsation". Tetrahedron 43 (21): 4875–4886. doi:10.1016/S0040-4020(01)87670-5.